
Explore our blog for helpful articles about UV safety, weather updates, and healthy outdoor living. Stay informed and make every sunny day a safe one!
Popular articles
Acid rain is one of the most pressing environmental issues, affecting ecosystems, infrastructure, and human health. But many people ask what causes acid rain and how it forms. Understanding its causes, chemical processes, and effects is crucial for reducing its impact and protecting our planet. In this post, we will explore what causes acid rain, the role of human activity, and some interesting facts and statistics about this environmental phenomenon.
What is Acid Rain?
Before answering what causes acid rain, it’s important to understand what acid rain is. Acid rain refers to any form of precipitation-rain, snow, sleet, or fog-that has an unusually low pH, typically below 5.6. This acidity results from chemical reactions in the atmosphere that produce sulfuric and nitric acids. Acid rain can harm plants, aquatic life, soils, and even human-made structures like buildings and statues.
What Causes Acid Rain?
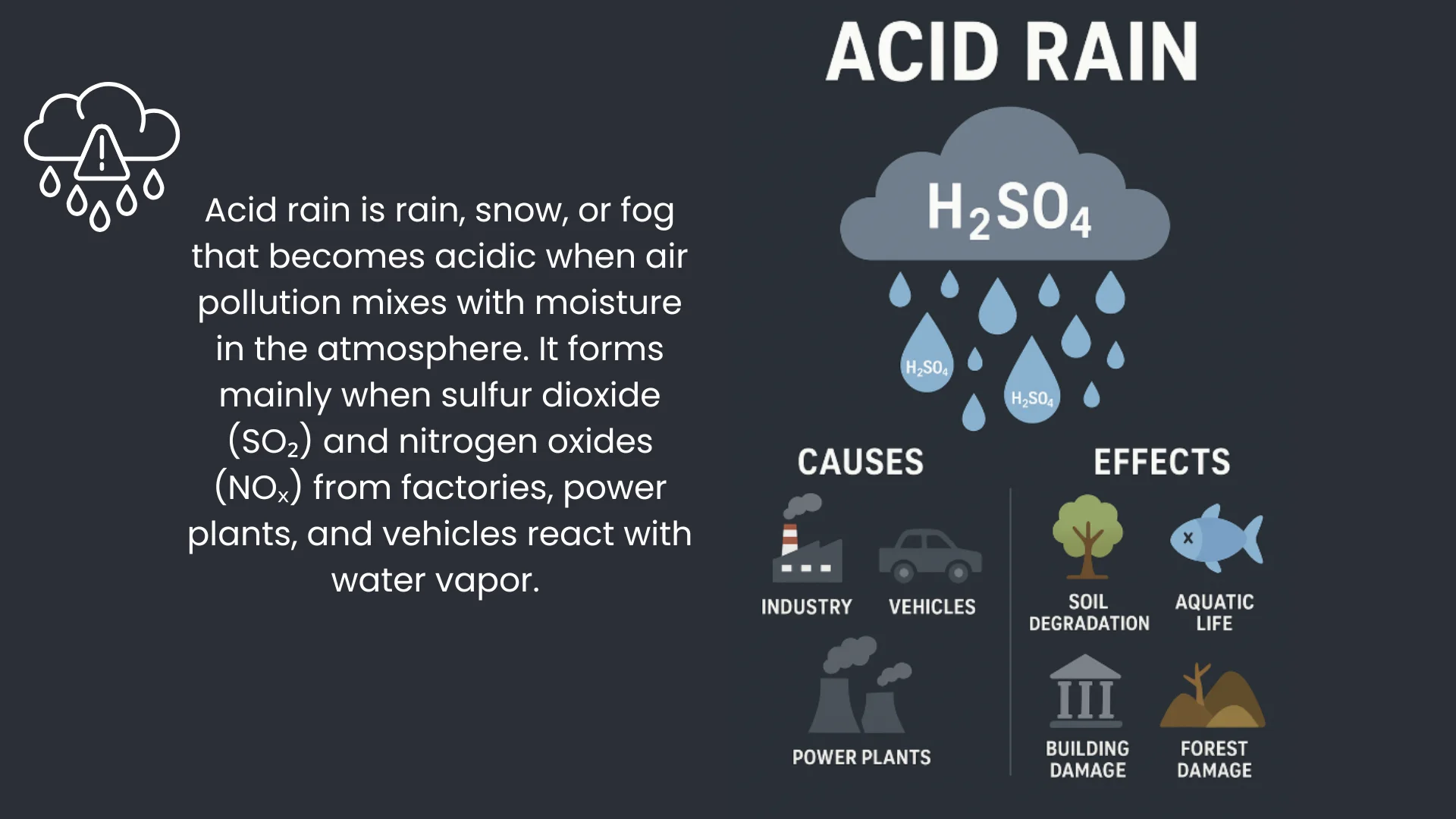
So, what causes acid rain? The process begins with the release of sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) into the atmosphere. These gases primarily originate from burning fossil fuels, such as coal, oil, and natural gas, in power plants, factories, and vehicles. Once in the atmosphere, these gases react with water, oxygen, and other chemicals to form acidic compounds.
The process that causes acid rain involves chemical reactions in the atmosphere: sulfur dioxide reacts with water and oxygen to form sulfuric acid (H₂SO₄), while nitrogen oxides react similarly to form nitric acid (HNO₃). These acids then mix with cloud moisture, eventually falling to the ground as acidic precipitation.
What Chemical Causes Acid Rain?
When people ask what chemical causes acid rain, the answer is primarily sulfuric acid and nitric acid. Sulfur dioxide (SO₂) is the main precursor for sulfuric acid, and nitrogen oxides (NOₓ) are the precursors for nitric acid. Both acids lower the pH of rainwater, making it harmful to the environment.
Natural vs. Human-Caused Acid Rain
Acid rain is not entirely a result of human activity. Natural sources, such as volcanic eruptions, lightning, and decaying vegetation, also release sulfur dioxide and nitrogen oxides into the atmosphere. However, the primary causes of acid rain are human activities, which significantly increase the concentration of these gases in the air.
For example, coal-fired power plants are major contributors, releasing large amounts of SO₂. Vehicles, particularly those using gasoline and diesel engines, emit nitrogen oxides. Industrial processes, such as smelting metals, also add to the problem. Because of this, acid rain is often more severe in industrialized regions.
What Are Some Causes of Acid Rain?
If you’re wondering what are some causes of acid rain, here’s a breakdown:
- Burning Fossil Fuels – Coal, oil, and natural gas combustion releases sulfur dioxide and nitrogen oxides into the atmosphere.
- Vehicle Emissions – Cars, trucks, and buses release nitrogen oxides, which contribute to nitric acid formation.
- Industrial Processes – Factories and power plants emit sulfur dioxide and other pollutants.
- Natural Sources – Volcanoes, forest fires, and lightning can release SO₂ and NOₓ naturally.
How Human Activity Contributes
The question what human activity causes acid rain to form is central to understanding its environmental impact. Industrialization, energy production, and transportation are the main contributors. Urban areas with high vehicle traffic and coal-powered electricity generation are particularly affected. Human activities accelerate the formation of acid rain far beyond natural levels, causing widespread ecological and structural damage.
What Are the Causes and Effects of Acid Rain?
While this article focuses on what causes acid rain, it is also important to understand its effects. Acid rain can:
- Damage Forests – Acidic precipitation weakens trees by leaching essential nutrients from the soil and damaging leaves.
- Harm Aquatic Life – Lakes and rivers become more acidic, threatening fish and other aquatic organisms.
- Corrode Buildings and Monuments – Acid rain reacts with limestone, marble, and metals, causing structural damage over time.
- Affect Human Health – Indirectly, acid rain can harm humans by contaminating water sources and increasing air pollution.
What Process Causes Acid Rain?
The process that causes acid rain involves several key steps:
- Emission of sulfur dioxide (SO₂) and nitrogen oxides (NOₓ) from natural and human sources.
- Chemical reactions with water, oxygen, and other atmospheric components to form sulfuric and nitric acids.
- Incorporation of these acids into cloud moisture.
- Precipitation of acidic water to the Earth’s surface as rain, snow, or fog.
This process demonstrates how interconnected human activities and natural chemical reactions are in creating acid rain.
What Are the Causes of Acid Rain in Different Regions?
Acid rain is more prevalent in industrialized areas where emissions are high. For example:
- Eastern United States – Coal-burning power plants historically contributed to severe acid rain affecting forests and lakes.
- Europe – Industrial regions in Germany, Poland, and the UK experienced extensive acid rain in the late 20th century.
- Asia – Rapid industrialization in China and India has increased acid rain in some regions, threatening agriculture and ecosystems.
Interesting Data and Facts About Acid Rain
- Acid rain was first identified as a significant environmental problem in the 19th century, but it became widely recognized in the 1970s and 1980s.
- The pH of normal rainwater is around 5.6 due to natural carbon dioxide in the atmosphere. Acid rain can have a pH as low as 4.0 or even lower in heavily polluted regions.
- Acid rain does not just fall near pollution sources; sulfur dioxide and nitrogen oxides can travel hundreds of miles in the atmosphere before forming acidic precipitation.
- Policies like the U.S. Clean Air Act and emissions trading programs have successfully reduced SO₂ and NOₓ emissions, decreasing acid rain in affected areas.
Protecting the Environment
Learning what causes acid rain is critical for environmental protection and public health. By understanding what causes acid rain?, what are causes of acid rain, and what human activity causes acid rain to form, we can develop strategies to reduce emissions and protect ecosystems.
Acid rain is a clear example of how human activity can alter natural processes, leading to environmental consequences. From damaging forests and lakes to corroding infrastructure, the impact of acid rain is widespread. However, with awareness, regulation, and cleaner energy alternatives, it is possible to reduce its occurrence and severity.
Whether you’re studying environmental science or simply curious about atmospheric phenomena, understanding what causes acid rain, what chemical causes acid rain, and what are the primary causes of acid rain? provides valuable insight into one of the most important environmental issues of our time.




